Abstract
Introduction: MM treatment has evolved substantially over the last decade, improving pt outcomes. (Rajkumar SV, 2016). Approvals of pomalidomide (POM), carfilzomib (CFZ), ixazomib, elotuzumab, and daratumumab have broadened options. The remitting-relapsing nature of MM and the availability of several drugs highlighted the need for a better understanding of treatment sequencing and its impact on outcomes. Optimization of sequence is important given that each successive line of treatment is met with diminishing effectiveness and a shorter period of disease response due to the nature of MM. In the US, lenalidomide (LEN) or bortezomib (BORT) and the combination of an immunomodulatory agent with a proteasome inhibitor (PI) are the most common treatments for pts with newly diagnosed MM (NDMM). Recent randomized phase 3 trials exploring novel combinations of drugs at relapse are limited by the characteristics of the pts enrolled in these studies and may not reflect clinical outcomes for real-world pts, especially in the US given practice patterns. Connect MM is the earliest and largest US-based prospective, observational, non-interventional, multicenter registry of pts with NDMM. We report the development of a novel model to test clinical outcomes of pts based on treatment sequencing between agents throughout the course of MM treatment using the Connect MM registry.
Methods: Between Sep 2009 and Dec 2011, 1493 pts were enrolled in Cohort 1 and between Dec 2012 and Apr 2016, 1518 pts were enrolled in Cohort 2, from 250 community and academic sites. Treatment transitions were analyzed at the time of disease progression as recorded by the treating physician. In this analysis, pts were grouped based on the agent/combination they were receiving prior to and after disease progression. All groups were mutually exclusive. The immunomodulatory agent group included LEN- and POM-containing regimens; the PI group included BORT- and CFZ-containing regimens; the immunomodulatory agent + PI group included combinations of LEN or POM with a PI; and the gap group included pts not receiving treatment for ≥90 days. As is common clinical practice, transitions could occur between any of the groups (eg, immunomodulatory agent to immunomodulatory agent + PI). Of particular interest were pts who 'switch class' of drugs at progression. Transitions were included for up to the sixth line of therapy and each pt could have more than 1 transition. Median and 3-y overall survival (OS) were estimated for each transition from the start of post-progression therapy using stratified covariate adjusted survival curves (Zhang, 2007). Covariates used included duration of pre-relapse therapy, age, and line of therapy where transition occurred.
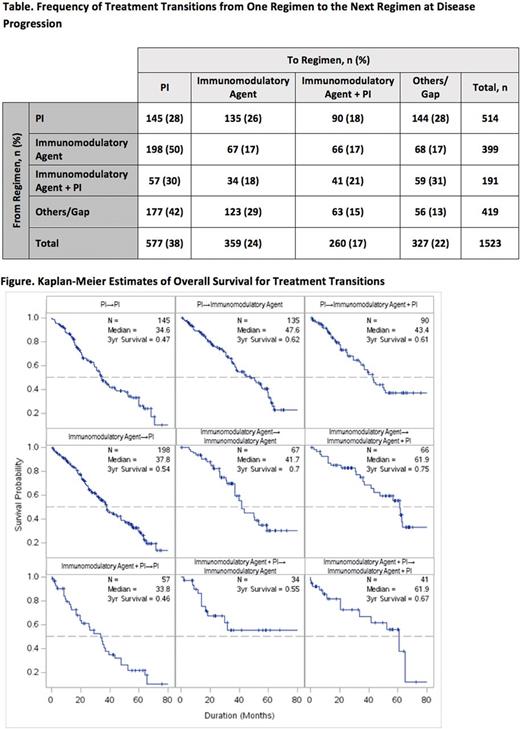
Results: This analysis included all pts from Cohorts 1 and 2 with a recorded treatment transition due to progression. A total of 1523 treatment transitions occurred in 856 pts. Fewer treatment transitions occurred with each subsequent line of therapy: 856 (line 1 to line 2); 385 (line 2 to line 3); 175 (line 3 to line 4); and lower numbers for subsequent lines. Numbers of treatment transitions between groups are shown in the Table. Median OS was longest for pts who received an immunomodulatory agent regimen and transitioned to an immunomodulatory agent + PI regimen (n = 66 transitions; 61.9 mo) or received an immunomodulatory agent + PI regimen and transitioned to another immunomodulatory agent + PI regimen (n = 41 transitions; 61.9 mo) (Figure). 3-y OS rates for these transitions were 75% and 67%, respectively. Outcomes for other transitions are shown in the Figure.
Conclusions: We devised a novel model that can study treatment transitions. To our knowledge, this is the first time treatment transitions have been documented in such detail in MM pts in a large prospective trial. This model allows us to study outcomes based on specific treatment sequences. In this analysis, the longest OS was observed for pts who received an immunomodulatory agent with or without a PI first and then transitioned to a regimen containing an immunomodulatory agent plus PI. The benefit from the continued use of immunomodulatory agents at the time of progression in this analysis could be from the persistent immune modulation function of these drugs even in pts who have experienced progression on an immunomodulatory agent based regimen. This model can provide useful information on optimal sequencing as more agents enter routine clinical practice.
Jagannath: Novartis: Consultancy; Medicom: Speakers Bureau; Celgene: Consultancy; Bristol-Meyers Squibb: Consultancy; MMRF: Speakers Bureau; Merck: Consultancy. Rifkin: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; McKesson Specialty Health: Employment; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gasparetto: Janssen, BMS, Celgene, Takeda: Honoraria; Janssen, BMS, Celgene: Consultancy; Celgene: Research Funding; Janssen, BMS, Celgene: Other: Travel, accommodations, or other expenses paid or reimbursed. Toomey: Celgene: Consultancy; Myriad Genetics: Speakers Bureau; Dava Oncology: Other: Travel. Durie: Takeda: Consultancy; Janssen: Consultancy. Hardin: Celgene: Consultancy. Terebelo: Celgene: Consultancy; Janssen: Speakers Bureau; Takeda: Speakers Bureau; Pharmacyclics: Speakers Bureau. Wagner: EveryFit: Consultancy; Gilead: Consultancy; Janssen: Consultancy. Narang: Celgene: Consultancy, Speakers Bureau; Janssen: Speakers Bureau. Srinivasan: Celgene: Employment. YoussefAgha: Celgene: Employment. Kitali: Celgene: Employment. Larkins: Celgene: Employment. Agarwal: Celgene Corporation: Employment. Abonour: Takeda: Other: Steering committee, Research Funding; Celgene: Other: Steering committee, Research Funding; Prothena: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal